The battery life of your next smartphone might be doubled or even tripled thanks to new technology in the works at Stanford. A group of researchers, including the former U.S. secretary of energy, has published breakthrough research that pushes beyond the limits of existing lithium ion batteries in the journal Nature Nanotechnology. The paper is titled “Interconnected hollow carbon nanospheres for stable lithium metal anodes.”
As it stands, lithium ion technology is a balancing act between safety (i.e. exploding batteries), energy storage density (how much charge a battery holds), and Coulombic efficiency, which measures how much usable energy a battery can transmit to a device as it discharges compared to how much energy it takes to charge up.
To improve that balance, the Stanford team covered a traditional lithium metal anode with a protective coat of carbon nanospheres, which mitigate the issue of “mossy metal deposits” that form over time and eventually lead to major safety concerns. While lithium metal is the “optimal choice,” balancing the three factors above gets tricky as these dendritic deposits form.
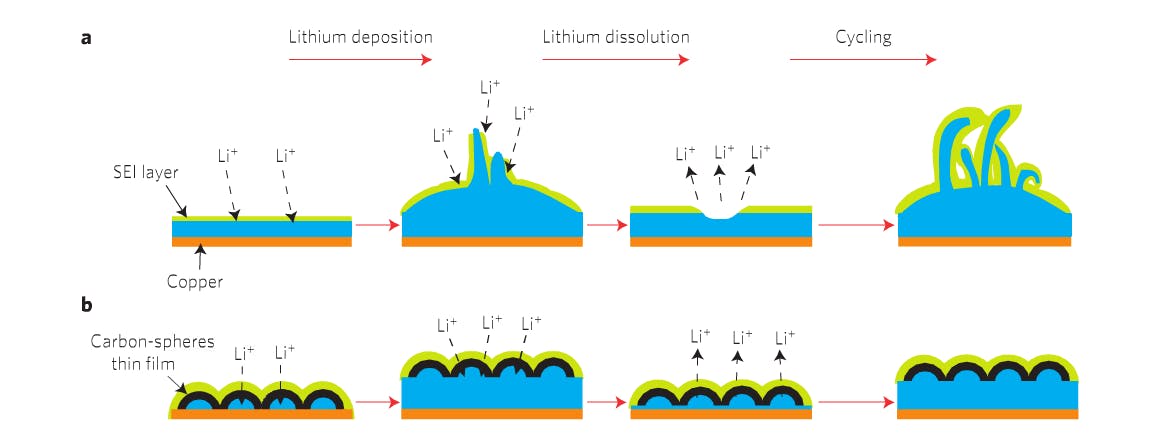
“In practical terms, if we can improve the capacity of batteries to, say, four times today’s, that would be exciting. You might be able to have cell phones with double or triple the battery life,” explains Stanford Professor Yi Cui. “Of all the materials that one might use in an anode, lithium has the greatest potential. Some call it the Holy Grail. It is very lightweight and it has the highest energy density. You get more power per volume and weight, leading to lighter, smaller batteries with more power.”
In their paper, the researchers note the potential applications for extended battery life technology in portable consumer electronics as well as electric cars and grid storage.
H/T Phys.org | Photo via sleepyneko/ Flickr (CC BY-SA 2.0)


